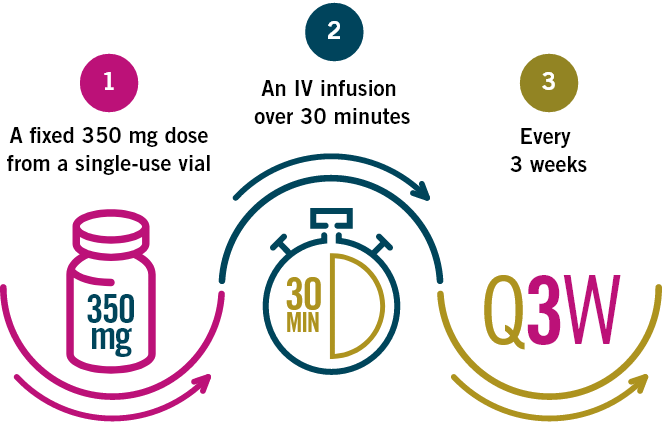
Treatment may be continued until symptomatic
disease progression or unacceptable toxicity.
In patients with a low body weight, a dose of 3 mg/kg every two weeks administered as an IV infusion over 30 minutes may be considered.
No dose reductions are recommended. Dosing delay or discontinuation may be required based on individual safety and tolerability.
Please see the Product Monograph for completing dosing and administration recommendations.
IV: intravenous.
LIBTAYO is for single use only. Dispose of any unused medicinal product or waste material in accordance with local requirements.
No compatibility studies have been performed. Do not mix with other medicinal products.
Once prepared, administer the diluted solution immediately. If diluted solution is not administered immediately, it may be stored temporarily either:

Learn about the safety and tolerability profile of LIBTAYO in patients with advanced CSCC.
Learn more
Explore the efficacy profile of LIBTAYO, including cases seen from the trials.
Learn more
Learn the details behind Study 1423 and Study 1540, two clinical trials used to evaluate the efficacy and safety profile of LIBTAYO.
Learn more